Max was only a toddler when his parents noticed there was “something different” about the way he moved. He was slower than other kids his age, and he struggled to jump. He couldn’t run.
Blood tests suggested he might have a genetic disease— one that affected a key muscle protein. Max’s dad, Tao Wang, a researcher for a climate philanthropy organization, says he and his wife were initially in denial. It took them a few months to take Max for the genetic test that confirmed their fears: he had Duchenne muscular dystrophy.
Duchenne is a rare disease that tends to affect young boys. It’s progressive—those affected generally lose muscle function as they get older. There is no cure. Many people with the disorder require wheelchairs by the time they reach their 20s. Most do not survive beyond their 30s.
Max’s diagnosis hit Wang and his wife “like a tornado,” he says. But eventually one of his doctors mentioned a clinical trial that he was eligible for. The trial was for an experimental gene therapy designed to replace the missing muscle protein with a shortened, engineered version that might help slow his decline or even reverse it. Enrolling Max in the trial was a no-brainer for Wang. “We were willing to try anything that could change the course [of the disease] and give us some hope,” he says.
That was more than two years ago. Today, Max is an active eight-year-old, says Wang. He runs, jumps, climbs stairs without difficulty, and even enjoys hiking. “He’s a totally different kid,” says Wang.
The gene therapy he received was recently considered for accelerated approval by the US Food and Drug Administration. Such approvals, reserved for therapies targeting serious conditions that lack existing treatments, require less clinical trial data than standard approvals.
While the process can work well, it doesn’t always. And in this case, the data is not particularly compelling. The drug failed a randomized clinical trial—it was found to be no better than a placebo.
Still, many affected by Duchenne are clamoring for access to the treatment. At an FDA advisory committee meeting in May set up to evaluate its merits, multiple parents of children with Duchenne pleaded with the organization to approve the drug immediately—months before the results of another clinical trial were due. On June 22, the FDA granted conditional approval for the drug for four- and five-year-old boys.
This drug isn’t the only one to have been approved on weak evidence. There has been a trend toward lowering the bar for new medicines, and it is becoming easier for people to access treatments that might not help them—and could harm them. Anecdotes appear to be overpowering evidence in decisions on drug approval. As a result, we’re ending up with some drugs that don’t work.
We urgently need to question how these decisions are made. Who should have access to experimental therapies? And who should get to decide? Such questions are especially pressing considering how quickly biotechnology is advancing. Recent years have seen an explosion in what scientists call “ultra-novel” therapies, many of which involve gene editing. We’re not just improving on existing classes of treatments—we’re creating entirely new ones. Managing access to them will be tricky.
Just last year, a woman received a CRISPR treatment designed to lower her levels of cholesterol—a therapy that directly edited her genetic code. Also last year, a genetically modified pig’s heart was transplanted into a man with severe heart disease. Debates have raged over whether he was the right candidate for the surgery, since he ultimately died.
For many, especially those with severe diseases, trying an experimental treatment may be better than nothing. That’s the case for some people with Duchenne, says Hawken Miller, a 26-year-old with the condition. “It’s a fatal disease,” he says. “Some people would rather do something than sit around and wait for it to take their lives.”
Expanding access
There’s a difficult balance to be reached between protecting people from the unknown effects of a new treatment and enabling access to something potentially life-saving. Trying an experimental drug could cure a person’s disease. It could also end up making no difference, or even doing harm. And if companies struggle to get funding following a bad outcome, it could delay progress in an entire research field—perhaps slowing future drug approvals.
In the US, most experimental treatments are accessed through the FDA. Starting in the 1960s and ’70s, drug manufacturers had to prove to the agency that their products actually worked, and that the benefits of taking them would outweigh any risks. “That really closed the door on patients’ being able to access drugs on a speculative basis,” says Christopher Robertson, a specialist in health law at Boston University.
It makes sense to set a high bar of evidence for new medicines. But the way you weigh risks and benefits can change when you receive a devastating diagnosis. And it wasn’t long before people with terminal illnesses started asking for access to unapproved, experimental drugs.
“If … somebody gets compassionate use and then something bad happens to them, investors run away. It’s a business risk.”
Alison Bateman-House, ethicist
In 1979, a group of people with terminal cancer and their spouses brought a legal case against the government to allow them to access an experimental treatment. While a district court ruled that one of the plaintiffs should be allowed to buy the drug, it concluded that whether a person’s disease was curable or not was beside the point—everyone should still be protected from ineffective drugs. The decision was eventually backed by the Supreme Court. “Even for terminally ill patients, there’s still a concept of safety and efficacy under the statute,” says Robertson.
Today, there are lots of ways people might access experimental drugs on an individual basis. Perhaps the most obvious way is by taking part in a clinical trial. Early-stage trials typically offer low doses to healthy volunteers to make sure new drugs are safe before they are offered to people with the condition the drugs are ultimately meant to treat. Some trials are “open label,” where everyone knows who is getting what. The gold standard is trials that are randomized, placebo controlled, and blinded: some volunteers get the drug, some get the placebo, and no one—not even the doctors administering the drugs—knows who is getting what until after the results have been collected. These are the kinds of studies you need to do to tell if a drug is really going to help people.
But clinical trials aren’t an option for everyone who might want to try an unproven treatment. Trials tend to have strict criteria about who is eligible depending on their age and health status, for example. Geography and timing matter, too—a person who wants to try a certain drug might live too far from where the trial is being conducted, or might have missed the enrollment window.
Instead, such people can apply to the FDA under the organization’s expanded access program, also known as “compassionate use.” The FDA approves almost all such requests. It then comes down to the drug manufacturer to decide whether to sell the person the drug at cost (it is not allowed to make a profit), offer it for free, or deny the request altogether.
Another option is to make a request under the Right to Try Act. The law, passed in 2018, establishes a new route for people with life-threatening conditions to access experimental drugs—one that bypasses the FDA. Its introduction was viewed by many as a political stunt, given that the FDA has rarely been the barrier to getting hold of such medicines. Under Right to Try, companies still have the choice of whether or not to provide the drug to a patient.
When a patient is denied access through one of these pathways, it can make headlines. “It’s almost always the same story,” says Alison Bateman-House, an ethicist who researches access to investigational medical products at New York University’s Grossman School of Medicine. In this story, someone is fighting for access to a drug and being denied it by “cold and heartless” pharma or the FDA, she says. The story is always about “patients valiantly struggling for something that would undoubtedly help them if they could just get to it.”
But in reality, things aren’t quite so simple. When companies decide not to offer someone a drug, you can’t really blame them for making that decision, says Bateman-House. After all, the people making such requests are usually incredibly ill. If someone were to die after taking that drug, not only would it look bad, but it could also put off investors from funding further development. “If you have a case in the media where somebody gets compassionate use and then something bad happens to them, investors run away,” says Bateman-House. “It’s a business risk.”
FDA approval of a drug means it can be sold and prescribed—crucially, it’s no longer experimental. Which is why many see approval as the best way to get hold of a promising new treatment.
As part of a standard approval process, which should take 10 months or less, the FDA will ask to see clinical trial evidence that the drug is both safe and effective. Collecting this kind of evidence can be a long and expensive process. But there are shortcuts for desperate situations, such as the outbreak of covid-19 or rare and fatal diseases—and for serious diseases with few treatment options, like Duchenne.
Anecdotes vs. evidence
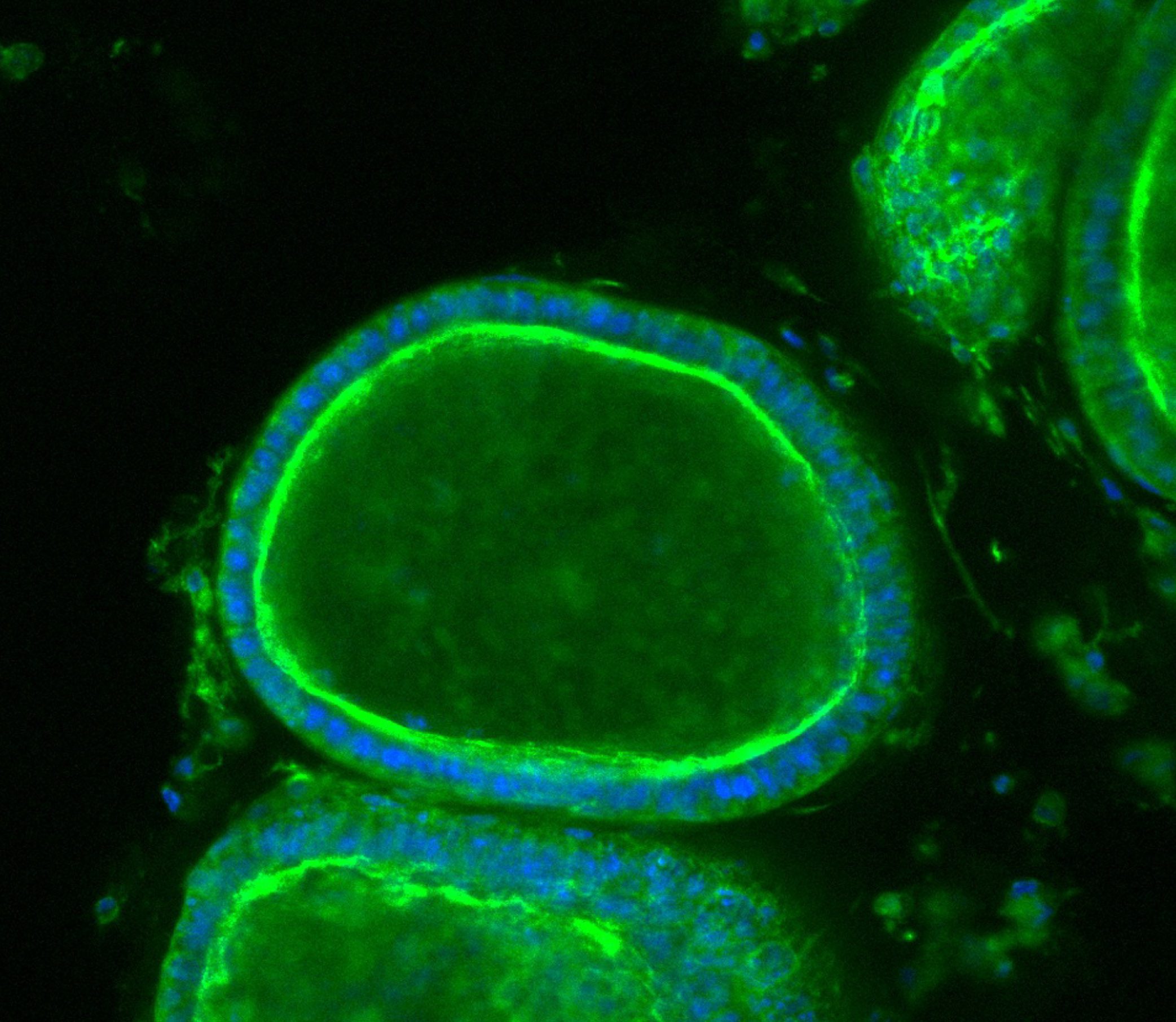
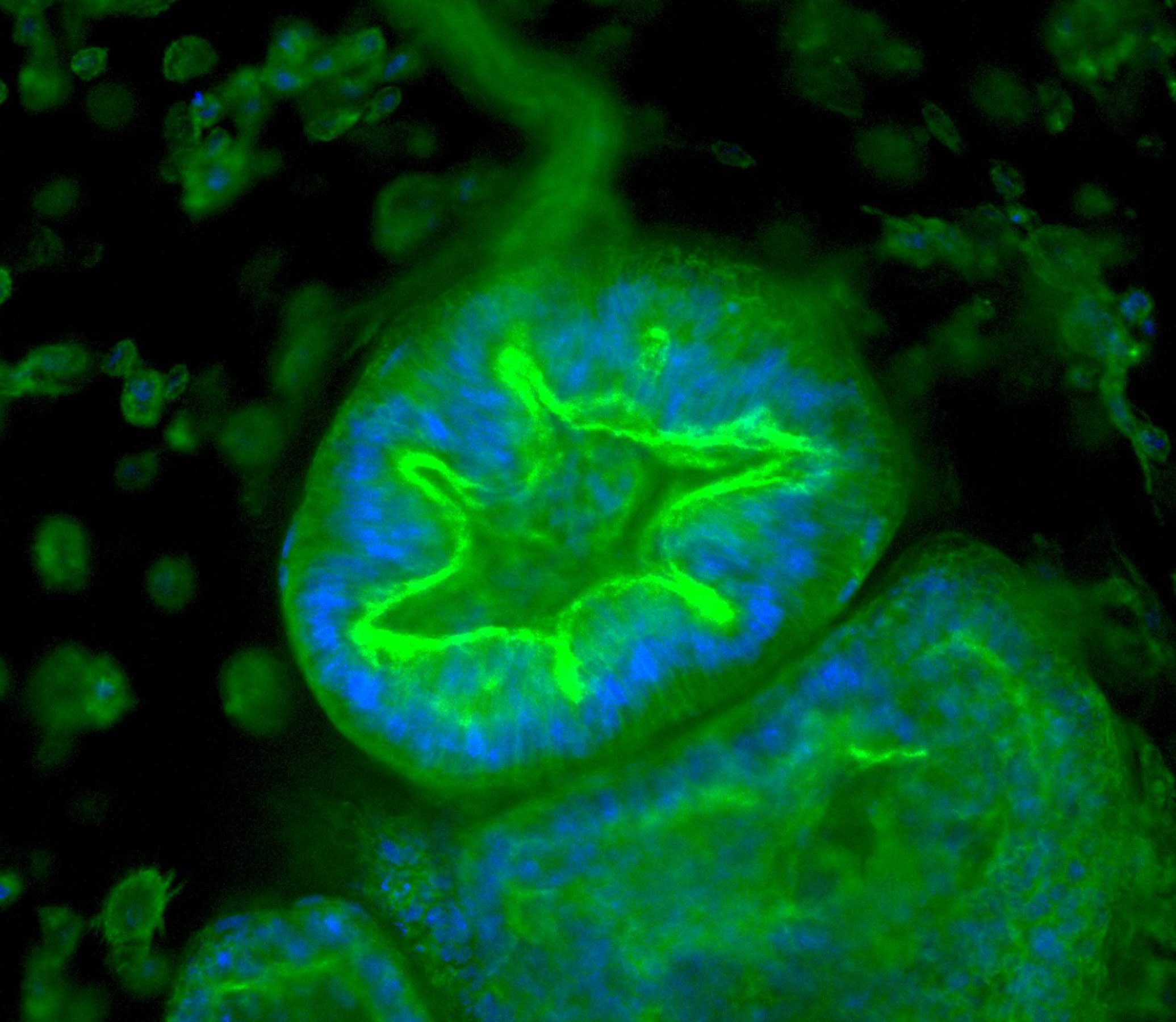
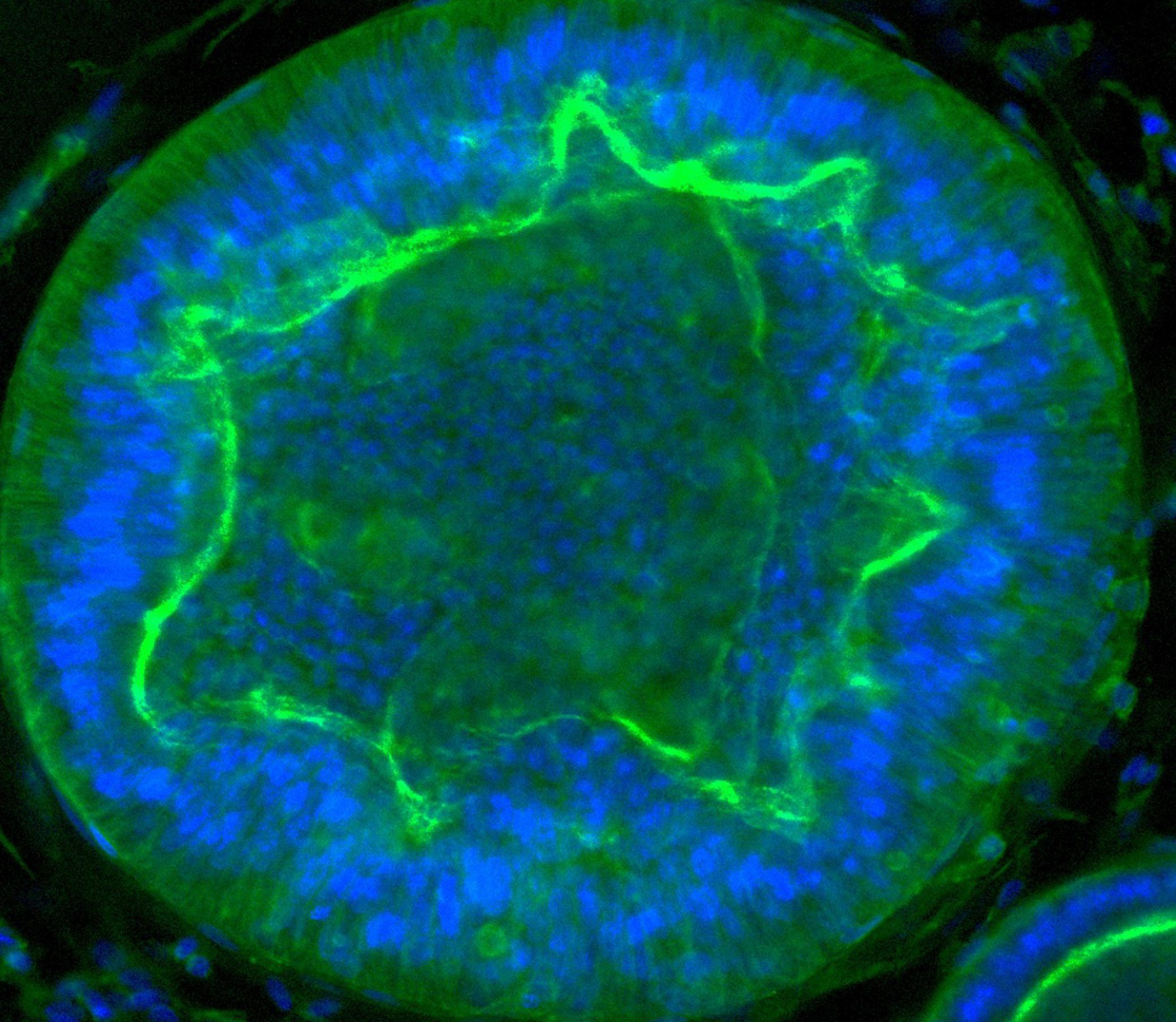
Max accessed his drug through a clinical trial. The treatment, then called SRP-9001, was developed by the pharmaceutical company Sarepta and is designed to replace dystrophin, the protein missing in children with Duchenne muscular dystrophy. The protein is thought to protect muscle cells from damage when the muscles contract. Without it, muscles become damaged and start to degenerate.
The dystrophin protein has a huge genetic sequence—it’s too long for the entire thing to fit into a virus, the usual means of delivering new genetic material into a person’s body. So the team at Sarepta designed a shorter version, which they call micro-dystrophin. The code for the protein is delivered by means of a single intravenous infusion.
The company’s initial goal was to develop it as a therapy for children between four and seven with a diagnosis of Duchenne. And it had a way to potentially fast-track the process.
Usually, before a drug can be approved, it will go through several clinical trials. But accelerated approval offers a shortcut for companies that can show that their drug is desperately needed, safe, and supported by compelling preliminary evidence.
For this kind of approval, drug companies don’t need to show that a treatment has improved anyone’s health—they just need to show improvement in some biomarker related to the disease (in Sarepta’s case, the levels of the micro-dystrophin protein in people’s blood).
There’s an important proviso: the company must promise to continue studying the drug, and to provide “confirmatory trial evidence.”
This process can work well. But in recent years, it has been a “disaster,” says Diana Zuckerman, president of the National Center for Health Research, a nonprofit that assesses research on health issues. Zuckerman believes the bar of evidence for accelerated approval has been dropping.
Many drugs approved via this process are later found ineffective. Some have even been shown to leave people worse off. For example, between 2009 and 2022, 48 cancer drugs received accelerated approval to treat 66 conditions—and 15 of those approvals have since been withdrawn.
Melfulfen was one of these. The drug was granted accelerated approval for multiple myeloma in February 2021. Just five months later, the FDA issued an alert following the release of trial results suggesting that people taking the drug had a higher risk of death. In October 2021, the company that made the drug announced it was to be taken off the market.
There are other examples. Take Makena, a treatment meant to reduce the risk of preterm birth. The drug was granted accelerated approval in 2011 on the basis of results from a small trial. Larger, later studies suggested it didn’t work after all. Earlier this year, the FDA withdrew approval for the drug. But it had already been prescribed to hundreds of thousands of people—nearly 310,000 women were given the drug between 2011 and 2020 alone.
And then there’s Aduhelm. The drug was developed as a treatment for Alzheimer’s disease. When trial data was presented to an FDA advisory committee, 10 of 11 panel members voted against approval. The 11th was uncertain. There was no convincing evidence that the drug slowed cognitive decline, the majority of the members found. “There was not any real evidence that this drug was going to help patients,” says Zuckerman.
Despite that, the FDA gave Aduhelm accelerated approval in 2021. The drug went on the market at a price of $56,000 a year. Three of the committee members resigned in response to the FDA’s approval. And in April 2022, the Centers for Medicare & Medicaid Services announced that Medicare would only cover treatment that was administered as part of a clinical trial. The case demonstrates that accelerated approval is no guarantee a drug will become easier to access.
The other important issue is cost. Before a drug is approved, people might be able to get it through expanded access—usually for free. But once the drug is approved, many people who want it will have to pay. And new treatments—especially gene therapies—don’t tend to be cheap. We’re talking hundreds of thousands, or even millions, of dollars. “No patient or families should have to pay for a drug that’s not proven to work,” says Zuckerman.
What about SRP-9001? On May 12, the FDA held an advisory committee meeting to assess whether the data supported accelerated approval. During the nine-hour virtual meeting, scientists, doctors, statisticians, ethicists, and patient advocates presented the data collected so far, and shared their opinions.
Sarepta had results from three clinical trials of the drug in boys with Duchenne. Only one of the three—involving 41 volunteers aged four to seven—was randomized, blinded, and placebo controlled.
Scientists will tell you that’s the only study you can draw conclusions from. And unfortunately, that trial did not go particularly well—by the end of 48 weeks, the children who got the drug were not doing any better than those who got a placebo.
But videos presented by parents whose children had taken the drug told a different story.
Take the footage shared by Brent Furbee. In a video clip taken before he got the gene therapy, Furbee’s son Emerson is obviously struggling to get up the stairs. He slowly swings one leg around while clinging to the banister, before dragging his other leg up behind him.
A second video, taken after the treatment, shows him taking the stairs one foot at a time, with the speed you’d expect of a healthy four-year-old. In a third, he is happily pedaling away on his tricycle. Furbee told the committee that Emerson, now six, could run faster, get up more quickly, and perform better on tests of strength and agility. “Emerson continues to get stronger,” he said.
It was one of many powerful, moving testimonies—and these stories appear to have influenced the FDA’s voting committee, despite many concerns raised about the drug.
The idea of providing the genetic code for the body to make a shortened version of dystrophin is based on evidence that people who have similarly short proteins have a much milder form of muscular dystrophy than those whose bodies produce little to no dystrophin. But it’s uncertain whether Sarepta’s protein, with its missing regions, will function in the same way.
Louise Rodino-Klapac, executive vice president, chief scientific officer, and head of R&D at Sarepta, defends the drug: “The totality of the evidence is what gives us great confidence in the therapy.” She has an explanation for why the placebo-controlled trial didn’t show a benefit overall. The groups of six- to seven-year-olds receiving the drug and the placebo were poorly matched “at baseline,” she says. She also says that the researchers saw a statistically significant result when they focused only on the four- and five-year-olds studied.
But the difference is not statistically significant for the results the trial was designed to collect. And there are some safety concerns. While most of the boys developed only “mild” side effects, like vomiting, nausea, and fever, a few experienced more serious, although temporary, problems. There were a total of nine serious complications among the 85 volunteers. One boy had heart inflammation. Another developed an immune disease that damages muscle fibers.
On top of all that, as things currently stand, receiving one gene therapy limits future gene therapy options. That’s because the virus used to deliver the therapy causes the body to mount an immune response. Many gene therapies rely on a type called adeno-associated virus, or AAV. If a more effective gene therapy that uses the same virus comes along in the coming years, those who have taken this drug won’t be able to take the newer treatment.
Despite all this, the committee voted 8–6 in favor of granting the drug an accelerated approval. Many committee members highlighted the impact of the stories and videos shared by parents like Brent Furbee.
“Now, I don’t know whether those boys got placebo or whether they got the drug, but I suspect that they got the drug,” a neurologist named Anthony Amato told the audience.
“Those videos, anecdotal as they are … are substantial evidence of effectiveness,” said committee member Donald B. Kohn, a stem-cell biologist.
The drugs don’t work?
Powerful as they are, individual experiences are just that. “If you look at the evidentiary hierarchy, anecdote is considered the lowest level of evidence,” says Bateman-House. “It’s certainly nowhere near clinical-trial-level evidence.”
This is not the way we should be approving drugs, says Zuckerman. And it’s not the first time Sarepta has had a drug approved on the basis of weak evidence, either.
The company has already received FDA approval to sell three other drugs for Duchenne, all of them designed to skip over faulty exons—bits of DNA that code for a protein. Such drugs should allow cells to make a longer form of a protein that more closely resembles dystrophin.
The first of these “exon-skipping” drugs, Exondys 51, was granted accelerated approval in 2016—despite the fact that the clinical trial was not placebo controlled and included only 12 boys. “I’ve never seen anything like it,” says Zuckerman. She points out that the study was far too small to be able to prove the drug worked. In her view, 2016 was “a turning point” for FDA approvals based on low-quality evidence—“It was so extreme,” she says.
Since then, three other exon-skipping drugs have received accelerated approval for Duchenne—two of them from Sarepta. A Sarepta spokesperson said a company-funded analysis showed that people with Duchenne who received Exondys 51 remained ambulatory longer and lived longer by 5.4 years—“data we would not have without that initial approval.”
But for many in the scientific community, that data still needs to be confirmed. “The clinical benefit still has not been confirmed for any of the four,” Mike Singer, a clinical reviewer in the FDA’s Office of Therapeutic Products, told the advisory committee in May.
“All of them are wanted by the families, but none of them have ever been proven to work,” says Zuckerman.
Will Roberts is one of the boys taking an exon-skipping drug—specifically, Sarepta’s Amondys 45. Now 10, he was diagnosed with Duchenne when he was just one year old. His treatment involves having a nurse come to his home and inject him every five to 10 days. And it’s not cheap. While his parents have a specialist insurance policy that shields them from the cost, the price of a year’s worth of treatment is around $750,000.
Will’s mother, Keyan Roberts, a teacher in Michigan, says she can’t tell if the drug is helping him. Last year he was running around in their backyard, but this year he needs a power chair to get around at school. “We definitely didn’t see any gains in ability, and it’s hard to tell if it made his decline … a little less steep,” Roberts says.
The treatment comes with risks, too. The Amondys 45 website warns that 20% of people who get the drug experience adverse reactions, and that “potentially fatal” kidney damage has been seen in people treated with a similar drug.
Roberts says she is aware of the risks that come with taking drugs like Amondys. But she and her husband, Ryan, an IT manager, were still hoping that SRP-9001 would be approved by the FDA. For the Robertses and parents like them, part of the desire is based on the hope, no matter how slim, that their child might benefit.
“We really feel strongly that we’re in a position now where we’re seeing [Will’s] mobility decline, and we’re nervous that … he might not qualify to take it by the time it’s made available,” she said in a video call, a couple of weeks after the advisory committee meeting.
Selling hope
On June 22, just over a month after the committee meeting, the FDA approved SRP-9001, now called Elevidys. It will cost $3.2 million for the one-off treatment, before any potential discounts. For the time being, the approval is restricted to four- and five-year-olds. It was granted with a reminder to the company to complete the ongoing trials and report back on the results.
Sarepta maintains that there is sufficient evidence to support the drug’s approval. But this drug and others have been made available—at eye-wateringly high prices—without the strong evidence we’d normally expect for new medicines. Is it ever ethical to sell a drug when we don’t fully know whether it will work?
I put this question to Debra Miller, mother of Hawken Miller and founder of CureDuchenne. Hawken was diagnosed when he was five years old. “The doctor that diagnosed him basically told us that he was going to stop walking around 10 years old, and he would not live past 18,” she says. “‘There’s no treatment. There’s no cure. There’s nothing you can do. Go home and love your child.’”
She set up CureDuchenne in response. The organization is dedicated to funding research into potential treatments and cures, and to supporting people affected by the disease. It provided early financial support to Sarepta but does not have a current financial interest in the company. Hawken, now a content strategist for CureDuchenne, has never been eligible for a clinical trial.
Debra Miller says she’s glad that the exon-skipping drugs were approved. From her point of view, it’s about more than making a new drug accessible.
“We all want hope. But in medicine, isn’t it better to have hope based on evidence rather than hope based on hype?”
Diana Zuckerman, president of the National Center for Health Research
“[The approvals] drove innovation and attracted a lot of attention to Duchenne,” she says. Since then, CureDuchenne has funded other companies exploring next-generation exon-skipping drugs that, in early experiments, seem to work better than the first-generation drugs. “You have to get to step one before you can get to step two,” she says.
Hawken Miller is waiting for the data from an ongoing phase 3 clinical trial of Elevidys. For the time being, “from a data perspective, it doesn’t look great,” he says. “But at the same time, I hear a lot of anecdotes from parents and patients who say it’s really helping a lot, and I don’t want to discount what they’re seeing.”
Results were due in September—just three months after the accelerated approval was granted. It might not seem like much of a wait, but every minute is precious to children with Duchenne. “Time is muscle” was the refrain repeated throughout the advisory committee meeting.
“I wish that we had the time and the muscle to wait for things that were more effective,” says Keyan Roberts, Will’s mom. “But one of the problems with this disease is that we might not have the opportunity to wait to take one of those other drugs that might be made available years down the line.”
Doctors may end up agreeing that a drug—even one that is unlikely to work—is better than nothing. “In the American psyche, that is the approach that [doctors and] patients are pushed toward,” says Holly Fernandez Lynch, a bioethicist at the University of Pennsylvania. “We have all this language that you’re ‘fighting against the disease,’ and that you should try everything.”
“I can’t tell you how many FDA advisory committee meetings I’ve been to where the public-comment patients are saying something like ‘This is giving me hope,’” says Zuckerman. “Sometimes hope helps people do better. It certainly helps them feel better. And we all want hope. But in medicine, isn’t it better to have hope based on evidence rather than hope based on hype?”
A desperate decision
A drug approved on weak data might offer nothing more than false hope at a high price, Zuckerman says: “It is not fair for patients and their families to [potentially] have to go into bankruptcy for a drug that isn’t even proven to work.”
The best way for people to access experimental treatments is still through clinical trials, says Bateman-House. Robertson, the health law expert, agrees, and adds that trials should be “bigger, faster, and more inclusive.” If a drug looks as if it’s working, perhaps companies could allow more volunteers to join the trial, for example.
Their reasoning is that people affected by devastating diseases should be protected from ineffective and possibly harmful treatments—even if they want them. Review boards assess how ethical clinical trials are before signing off on them. Participants can’t be charged for drugs they take in clinical trials. And they are carefully monitored by medical professionals during their participation.
That doesn’t mean people who are desperate for treatments are incapable of making good decisions. “They are stuck with bad choices,” says Fernandez Lynch.
This is also the case for ultra-novel treatments, says Robertson. At the start of trials, the best candidates for all-new experimental therapies may be those who are closer to death, he says: “It is quite appropriate to select patients who have less to lose, while nonetheless being sure not to exploit people who don’t have any good options.”
There’s another advantage to clinical trials. It’s hard to assess the effectiveness of a one-off treatment in any single individual. But clinical trials contribute valuable data that stands to benefit a patient community. Such data is especially valuable for treatments so new that there are few standards for comparison.
Hawken Miller says he would consider taking part in an Elevidys clinical trial. “I’m willing to take on some of that risk for the potential of helping other people,” he says. “I think you’ll find that in [most of the Duchenne] community, everyone’s very willing to participate in clinical trials if it means helping kids get cured faster.”
When it comes to assessing the likelihood that Elevidys will work, Will’s dad, Ryan Roberts, says he’s a realist. “We’re really close to approaching the last chance—the last years he’ll be ambulatory,” he says. For him as a dad, he says, the efficacy concerns aren’t relevant. “We will take the treatment because it’s going to be the only chance we have … We are aware that we’re not being denied a treatment that is a cure, or a huge game-changer. But we are willing to take anything we can get in the short window we have closing now.”