Ready, set, grow: These are the biotech plants you can buy now
This spring I am looking forward to growing some biotech in my backyard for the first time. It’s possible because of startups that have started selling genetically engineered plants directly to consumers, including a bright-purple tomato and a petunia that glows in the dark.
This week, for $73, I ordered both by pressing a few buttons online.
Biotech seeds have been a huge business for a while. In fact, by sheer mass, GMOs are probably the single most significant product of genetic engineering ever. Except most of us aren’t planting rows of cotton or corn that can resist worms or survive a spritz of RoundUp, the big gene-splicing innovations that companies like Monsanto and Pioneer Hi-Bred first introduced in the 1990s.
What makes these new plants different is that you can buy them directly from their creators and then plant them in the yard, on a balcony, or just in a pot.

Purple tomato
Starting off my biotech shopping spree, I first spent $20 to order 10 tomato seeds from Norfolk Health Produce, a small company in Davis, California, that created what it calls the Purple Tomato. The seeds have a gene introduced from a snapdragon flower, which adds a nutrient, anthocyanin, that also gives the fruits their striking color.
According to Channa S. Prakash, a geneticist and dean at Tuskegee University, the tomato is the “the first-of-its kind GMO food crop marketed directly to home gardeners.”
The CEO of the company, Nathan Pumplin, was packing seeds when I reached him by phone. He claimed that anthocyanin has health benefits—it’s an antioxidant—but he agreed that the color is a useful sales pitch.
“I don’t need to make a label that says this red tomato is better for you than the other red tomato,” says Pumplin. “We can simply put out the purple tomato, and people say, ‘Oh my gosh, this tomato is purple.’ Its beauty is a distinguishing characteristic that people can just immediately see and understand.”
There is a plan to mass-produce the purple tomatoes for sale in supermarkets. But Pumplin says the company couldn’t ignore thousands of requests from regular gardeners. “It’s not the main focus of our business, but we are very interested in having people grow these at home,” he says. And “if home gardeners want to save the seed and replant it in their gardens for their own use, that is okay.”
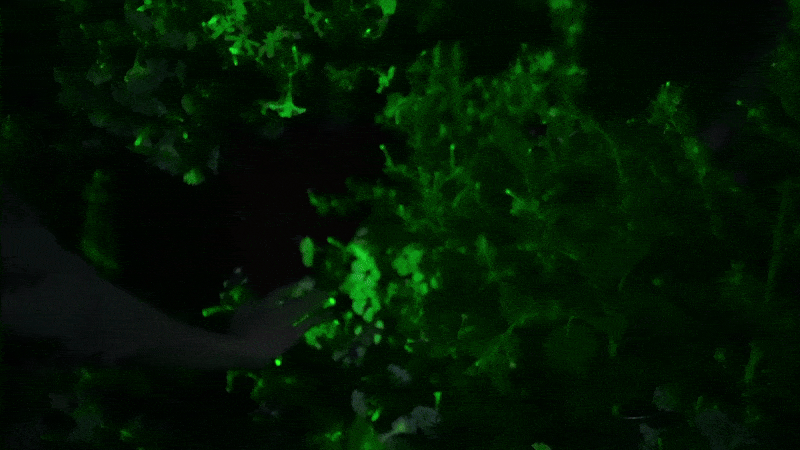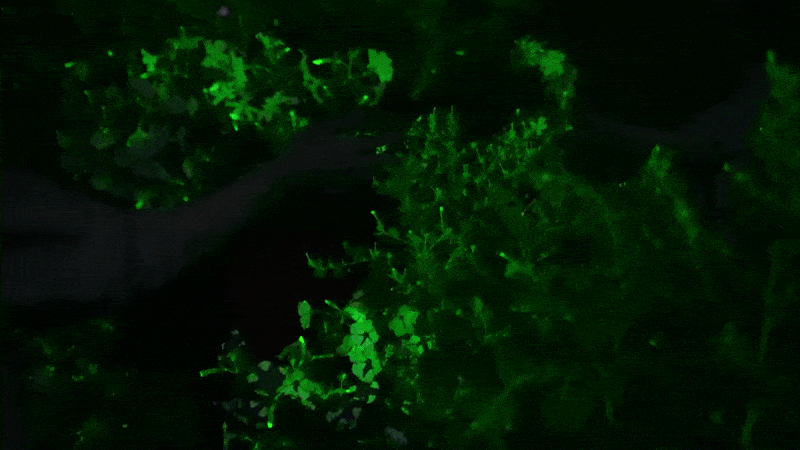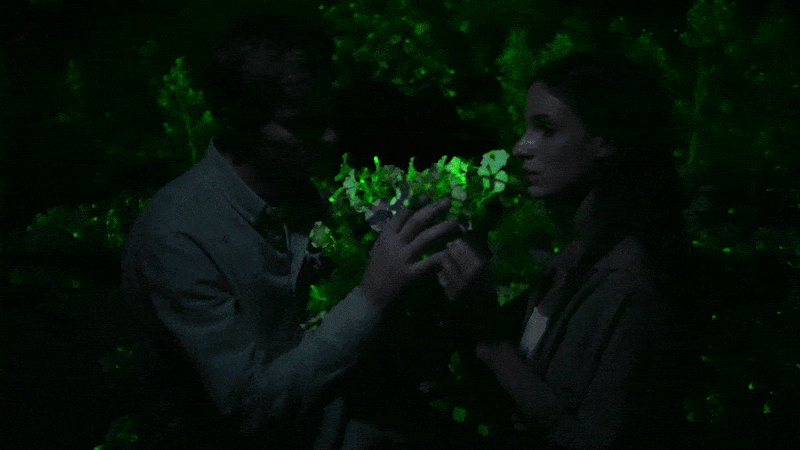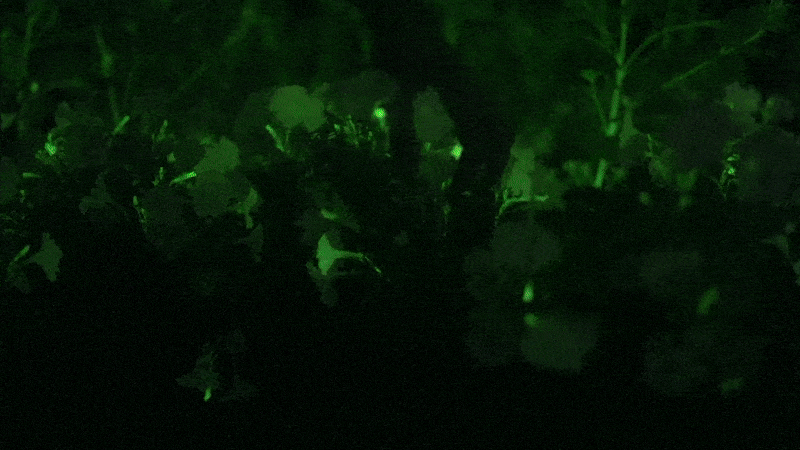


Glowing flower
I next decided to shell out for the “firefly petunia,” so called because the plant is supposed to glow in the dark. It’s sold by Light Bio, a startup backed by the venture capital firm NFX .
The plant is such a novelty that it’s being sold in a preorder, with promises they will arrive by May. One petunia plant costs $29 plus $24 for shipping. The company’s marketing promises that your plant will unveil “mesmerizing luminescence after dusk” and that “its soothing light is produced from living energy, cultivating a deeper connection with the inner life of the plant.”
Finally, “Your nurturing care will be rewarded with even greater brilliance.”
It joins a short list of ornamental plants with gene modifications. Another is an orange petunia, approved in the US in 2021, that got its unusual color from a corn gene. (When some copies got loose prior to approval, officials in the US and Europe demanded its eradication in what became known as “the petunia carnage.”)
Karen Sarkisyan, a synthetic biologist at the MRC Laboratory of Medical Sciences in the UK, is one of the petunia’s creators, and also the chief scientist of Light Bio. His lab is interested in using bioluminescence as a reporter system—a plant could reveal, for instance, how it responds to a toxin or viral infection in lab experiments.
“In general, we’re trying to make useful things, so this is more of an exception,” he says of the firefly petunia. “The motivation was more about merging biology and art, rather than utility.”
Like a lot of things in biotech, making a glowing petunia was not easy to do—it’s the seemingly sudden result of decades of research into the chemistry that permits certain plants and animals to glow faintly.
Imposing those genetic circuits on plants did not work too well at first. Several years ago, for instance, a Kickstarter project that raised nearly $500,000 to make glowing roses failed to deliver on its promises after the project proved too difficult.
“It was fairly obvious … that there was no good technology at that time,” says Sarkisyan, who later played a role in discovering genes from a glowing fungus which, after being added to a petunia, made it shine brightly enough to work as a novelty item.
That work continues. Sarkisyan says the company is working on “increasing the brightness and making more colors.” It’s also working on making other types of plants glow, although which ones remain a secret. “I cannot really comment on specific species we’re working on,” he says, although he did show me a photo of a spectacular glowing chrysanthemum.



Sarkisyan told me he sometimes likes to relax among the glowing plants and have a meditative experience. Ironically, he can only do that in the lab and not at home, since he lives in the UK. The country, which takes a stricter view on GMOs, has not approved the plants for sale (neither has Europe).
But he thinks the petunia could win over critics. “Especially with all the talk and concerns about the GM stuff, this is the first time there can be a safe, friendly, pleasant GM house plant in every home,” he says. “We think it’s a very interesting project because it is one of the first in consumer biotech. I do think we will see more and more in the future.”
My tomato seeds and glowing petunia haven’t arrived in the mail yet, and there’s still snow where I am. But come spring, I hope to be putting my first biotech crop in the ground.


